US Food and Drug Administration officials found multiple faults at a plant run by one of India’s biggest drugmakers as the watchdog continues to unearth wide-ranging lapses across the country’s factories while working through a pandemic-era inspection backlog.
Auditors uncovered “deficient” manufacturing equipment cleaning and storage controls during a visit last month to an Aurobindo Pharma Ltd. facility in Anakapalli, eastern India, according to a FDA report obtained by Bloomberg News through a Freedom of Information Act request. Sampling tools weren’t cleaned and maintained to prevent contamination at the plant, which produces certain active pharmaceutical ingredients — the raw materials core to making drugs.
Laboratory controls also didn’t include the establishment of scientifically sound and appropriate specification, designed to assure that drug products conform to appropriate standards of identity, quality and purity, the report said. During a warehouse walk-through the auditors also observed raw materials being stored in excessively hot and humid conditions and not compliant with label storage requirements, it said.
Read more: US Inspectors Find Safety Lapses at Indian Drugmakers
Aurobindo, India’s second-largest drugmaker by revenue, didn’t respond to a request for comment from Bloomberg News about the FDA’s report. In a statement last month, Aurobindo Pharma said the FDA recorded “procedural” observations following the inspection of its factory in Anakapalli in May. While the company didn’t give details on the observations, it said it was working to address them and will respond to the FDA.
The FDA typically waits to receive a company’s plan to address issues before deciding on next steps. The Anakapalli factory produces APIs for cardiovascular and anti-fungal treatments, according to a presentation to investors in August.
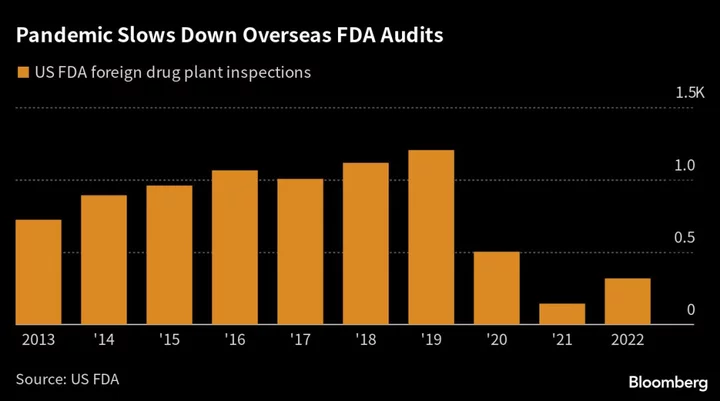
Indian drugmakers have faced a wave of adverse audits this year as the US agency races through more than 1,000 foreign drug-plant inspections that were missed due to travel restrictions during Covid. Despite an uptick in 2022, factory visits were still down 74% from 2019’s level.
India’s $50 billion drug-making industry, which Prime Minister Narendra Modi has championed as the “pharmacy of the world”, is also under heightened scrutiny after a number manufacturing scandals, including the deaths of dozens of children in Gambia and Uzbekistan from adulterated cough syrup.
The problems in Indian factories have rippled to the US, which largely depends on supplies of cheap generic drugs from the South Asian nation. Plant shutdowns, recalls and extra testing has seen the number of medications in short supply hit a five-year high. But the inspection reports from the FDA signal the shortage may worsen, ramping up pressure on the White House task force formed to tackle quality problems in the supply chain.
Read more: More Quality Issues Plague Firm Behind Cancer Drug Shortage
Headquartered in the Indian pharmaceutical hub of Hyderabad, Aurobindo was founded in 1986 by P.V. Ramprasad Reddy and K. Nityananda Reddy as a maker of semi-synthetic penicillin. It has since expanded to become an exporter of drugs to more than 150 countries and makes 90% of its roughly $3 billion annual revenue from international markets. It gets about 15% of its sales from APIs.
The firm has previously been told to correct issues. In January last year, its Doultabad plant in south-central India was given a warning letter, one of the agency’s strongest enforcement measures that can lead to import bans and delays in product approvals. The FDA said the facility produced adulterated API and that repeated failures “demonstrate that executive management oversight and control over the manufacture of drugs is inadequate.”
Aurobindo is making efforts to get the warning letter “cleared,” Chief Financial Officer Santhanam Subramanian said on a call with industry analysts last month.