ARLINGTON, Va.--(BUSINESS WIRE)--Aug 25, 2023--
Kerecis ® Shield™, the new fish-skin wound-treatment product family for private doctors’ clinics, has been approved by multiple leading Medicare Administrative Contractors (MACs). This means that physicians operating in private clinics will not need to submit invoices for reimbursement, improving efficiency by eliminating a step in the reimbursement process.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230825973824/en/
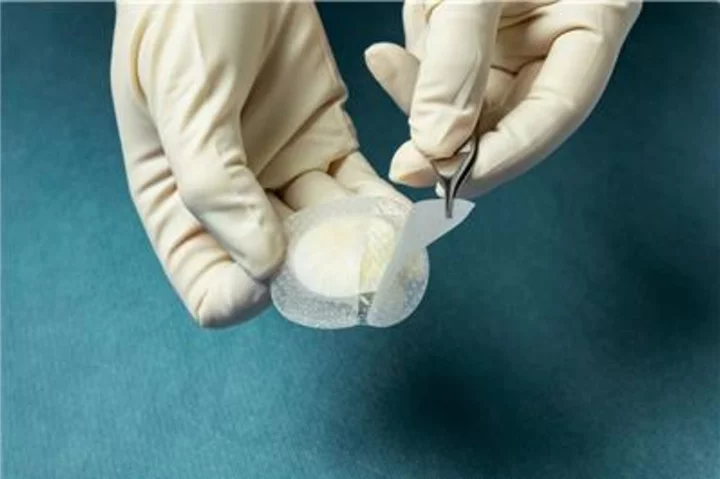
Multiple Medicare Administrative Contractors (MACs) have approved coverage for Kerecis Shield. The product integrates the Kerecis medical-fish-skin graft with a silicone contact layer to provide an advanced treatment for chronic and complex wounds. (Photo: Business Wire)
Kerecis is pioneering the use of sustainably sourced fish skin and fatty acids in cellular therapy, tissue regeneration and protection. The Kerecis Shield product line, which was announced earlier this year, integrates the Kerecis medical-fish-skin graft with a silicone contact layer to provide an advanced treatment for chronic and complex wounds. The flagship product, Shield Adhesive, features a silicone contact layer that overlaps the fish skin and helps holds the graft in place. The initial versions of Shield are available as 20mm and 30mm circular grafts.
“This rapid insurance coverage for our new Shield product line means that 42% of Medicare patients throughout the country will have easier access to the newest fish-skin treatment,” said Fertram Sigurjonsson, founder and CEO of Kerecis. “More than 150 million Americans have insurance coverage for our existing portfolio of medical-fish-skin products. This rapid coverage of our new Shield product will allow private clinics to give their patients the new, sophisticated treatments they need.”
About Kerecis
Kerecis develops products from fish skin and fatty acids for cellular therapy, tissue regeneration and protection. When grafted onto damaged human tissue or implanted, the patented material supports the body’s own processes to heal and regenerate. Because no disease-transfer risk exists between cold-water fish and humans, the Kerecis fish skin is only gently processed and retains its similarity to human tissue. The gentle processing preserves the skin’s original three-dimensional structure, maintaining its inherent natural strength, complexity and molecules (such as fatty acids). Clinical studies have found that the Kerecis products heal wounds faster than competing products. Kerecis is the only approved manufacturer of medical devices containing intact fish skin globally.
Kerecis is the fastest-growing and one of the top five companies in the U.S. biologics-skin and dermal-substitute market, according to SmartTRAK Business Intelligence. Kerecis’ expanding product portfolio includes SurgiBind ® /SurgiClose ®, which are used for reconstructive surgery in hospital operating rooms; GraftGuide ®, which is mostly sold to burn centers; and MariGen ® and Shield™, which are sold to healthcare facilities to treat diabetic and other chronic wounds.
Kerecis is committed to the United Nations Sustainable Development Goals. The fish skin used in Kerecis products derives from wild and sustainable fish stock caught in pristine Icelandic waters and processed with 100% renewable energy in the town of Isafjordur, close to the Arctic Circle. For more information, visit https://www.kerecis.com.
Trademarks and registered trademarks are the property of their respective owners.
View source version on businesswire.com:https://www.businesswire.com/news/home/20230825973824/en/
CONTACT: Kay Paumier
Communications Plus
kay@communicationsplus.net
Office: 408-370-1243
Mobile: 408-806-1177
KEYWORD: UNITED STATES NORTH AMERICA VIRGINIA
INDUSTRY KEYWORD: MEDICAL SUPPLIES HEALTH MEDICAL DEVICES STEM CELLS PRACTICE MANAGEMENT HEALTH INSURANCE BIOTECHNOLOGY
SOURCE: Kerecis
Copyright Business Wire 2023.
PUB: 08/25/2023 01:41 PM/DISC: 08/25/2023 01:42 PM
http://www.businesswire.com/news/home/20230825973824/en